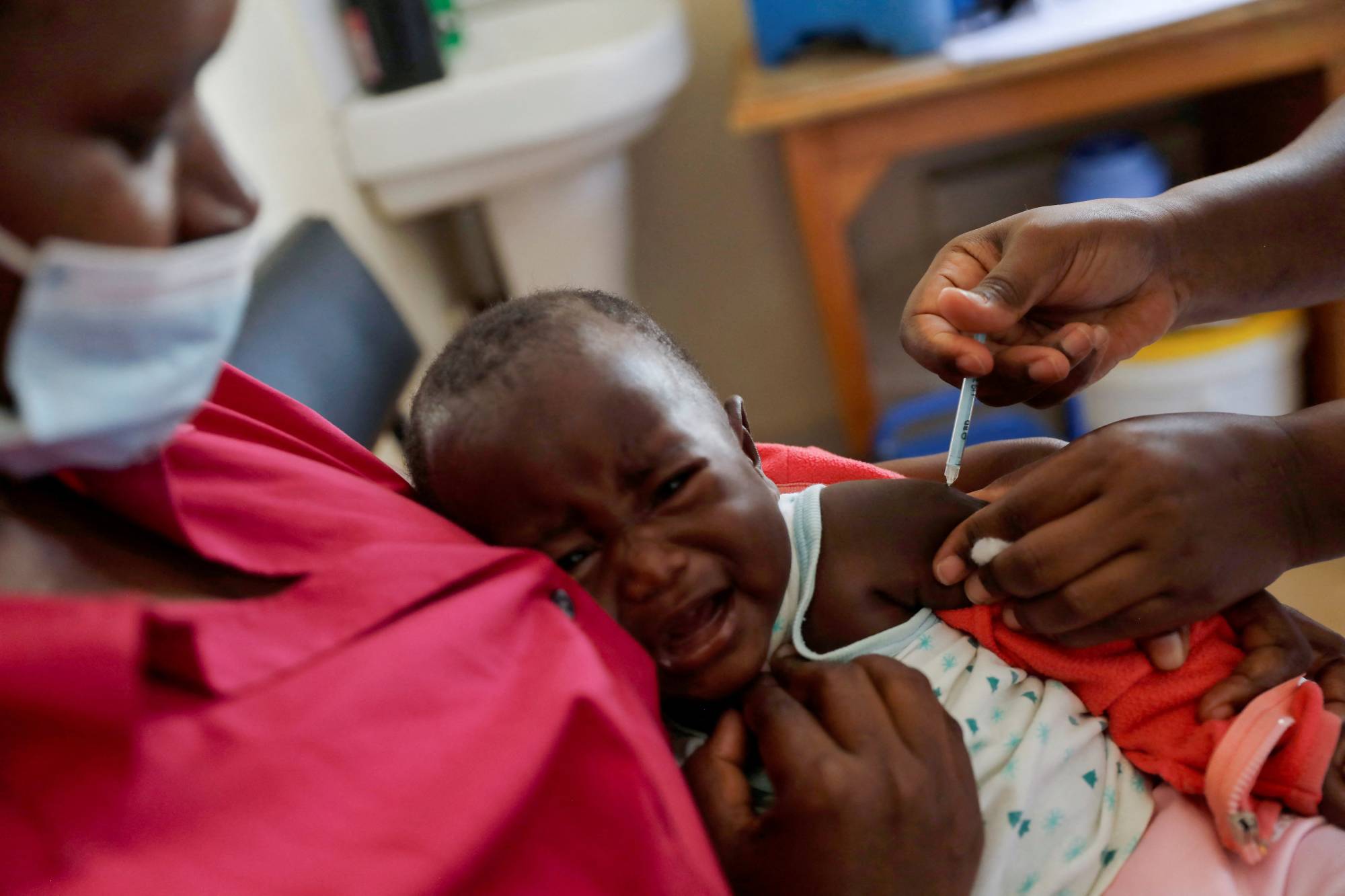
After decades of work, the World Health Organization endorsed the first-ever malaria vaccine last year — a historic milestone that promised to drive back a disease that kills a child every minute.
In reality, efforts are falling well short of that, with a lack of funding and commercial potential thwarting GSK PLC's capacity to produce as many doses of its shot as needed, according to interviews with about a dozen WHO officials, GSK staff, scientists and nonprofit groups.
The British drugmaker committed to produce up to 15 million doses every year through 2028, following 2019 pilot programs — considerably less than the WHO says is needed. It is currently unlikely to make more than a few million annually before 2026, according to a source close to the vaccine rollout.
















With your current subscription plan you can comment on stories. However, before writing your first comment, please create a display name in the Profile section of your subscriber account page.