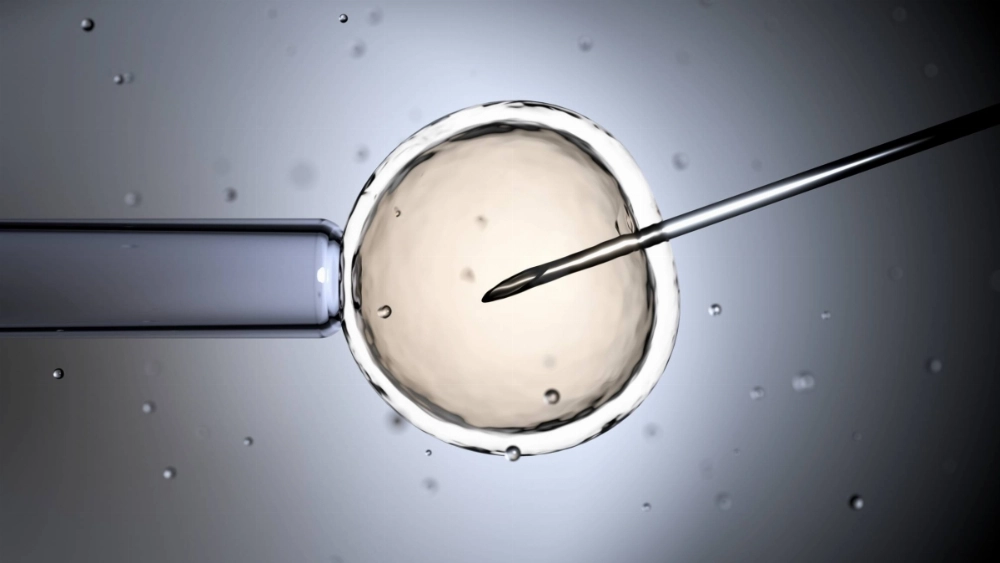
A team led by a professor at Osaka University's Bioinformatics Center has replicated the mouse embryo implantation process in laboratory equipment with a high success rate.
Masahito Ikawa and his team on Wednesday said that they had developed a method of culturing fragments of uterine tissue from mice in laboratory containers to achieve embryo implantation.
The team also discovered protein interactions that help drive embryo implantation.

















With your current subscription plan you can comment on stories. However, before writing your first comment, please create a display name in the Profile section of your subscriber account page.