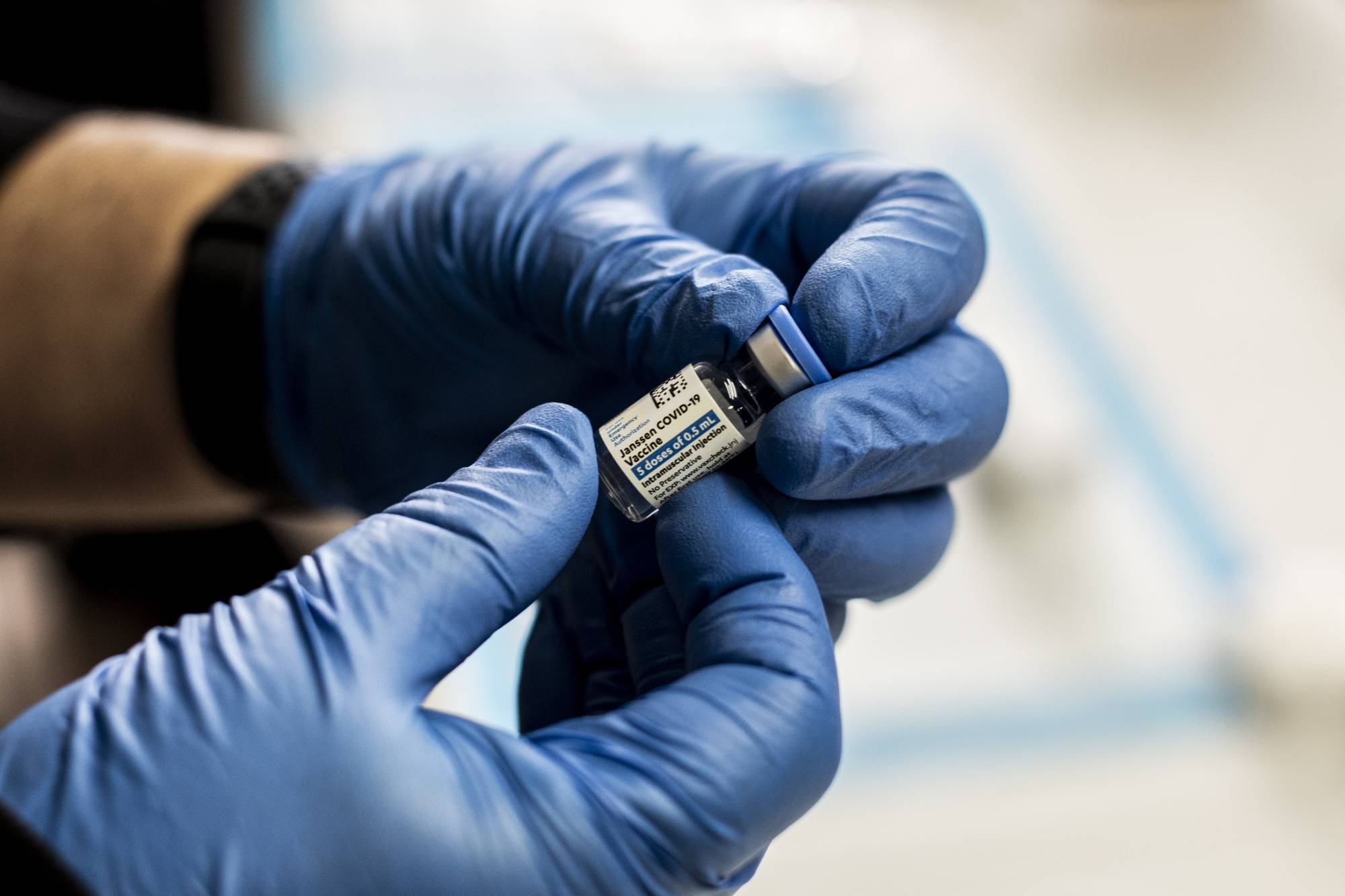
The United States can immediately resume use of Johnson & Johnson's COVID-19 vaccine, top health regulators said on Friday, ending a 10-day pause to investigate its link to extremely rare but potentially deadly blood clots.
The Centers for Disease Control and Prevention (CDC) and Food and Drug Administration said the risks of experiencing the syndrome involving severe blood clots and low platelets as a result of the vaccine was very low. They found 15 cases in the 8 million shots given.
"We are no longer recommending a pause in the use of this vaccine," CDC Director Rochelle Walensky told a news briefing. "Based on the in-depth analysis, there is likely an association but the risk is very low."


















With your current subscription plan you can comment on stories. However, before writing your first comment, please create a display name in the Profile section of your subscriber account page.