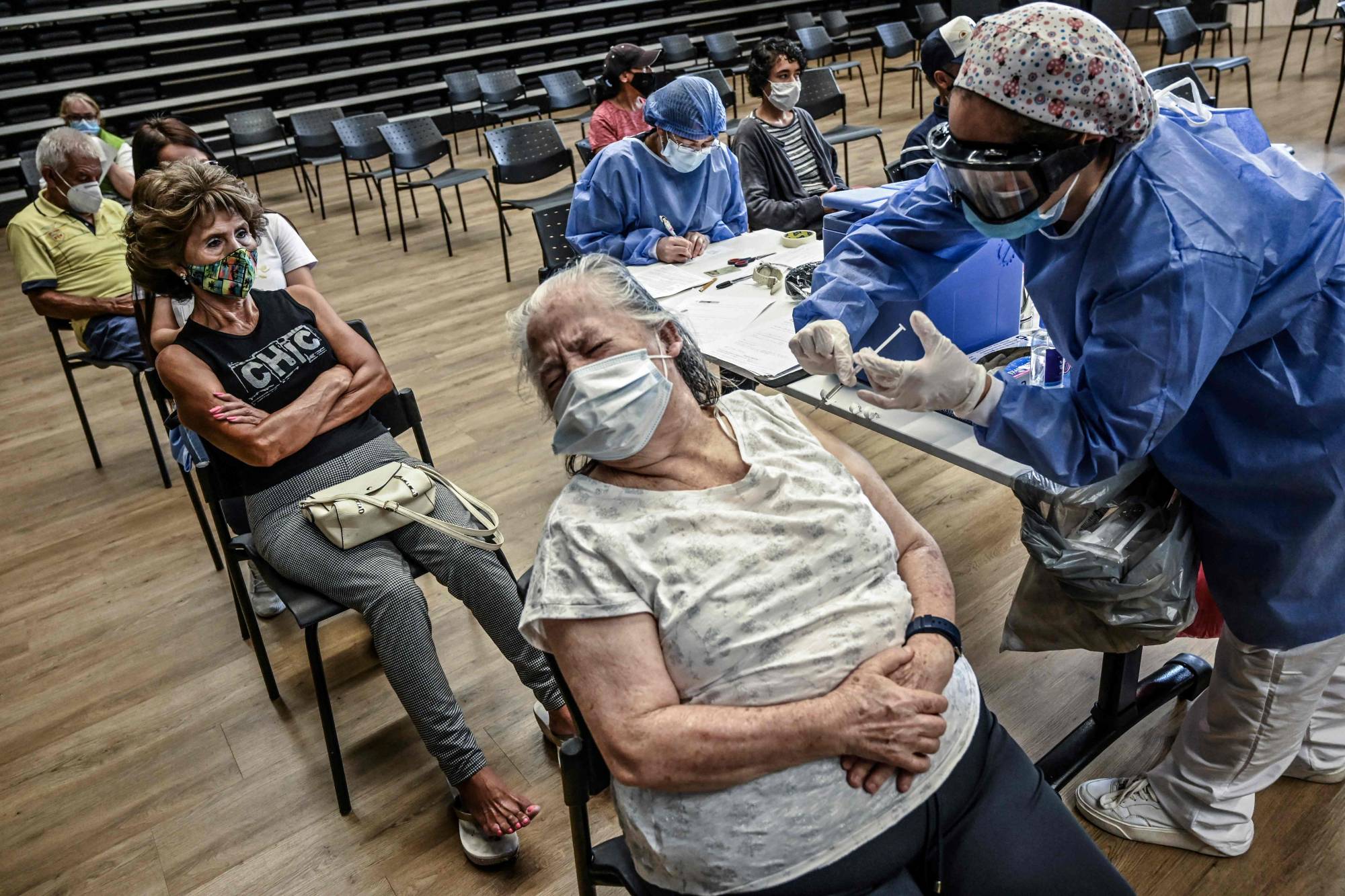
Growing worries that AstraZeneca PLC’s COVID-19 vaccine causes rare blood clots could hinder immunization campaigns across the world, from London to Seoul.
Reviews by U.K. and European Union regulators finding potential links to the unusual side effects are another blow for the shot, a cheaper and easier-to-deploy product that many nations are counting on in a bid to end the pandemic.
Safety concerns following increasing reports of blood clots in people who received the inoculation could shake confidence in it, even though regulators have agreed that the benefits outweigh the risks. Although many regions are turning their attention to vaccines from Johnson & Johnson and developers in China, Russia and elsewhere, they are in a difficult position with demand for doses far outstripping supply.

















With your current subscription plan you can comment on stories. However, before writing your first comment, please create a display name in the Profile section of your subscriber account page.