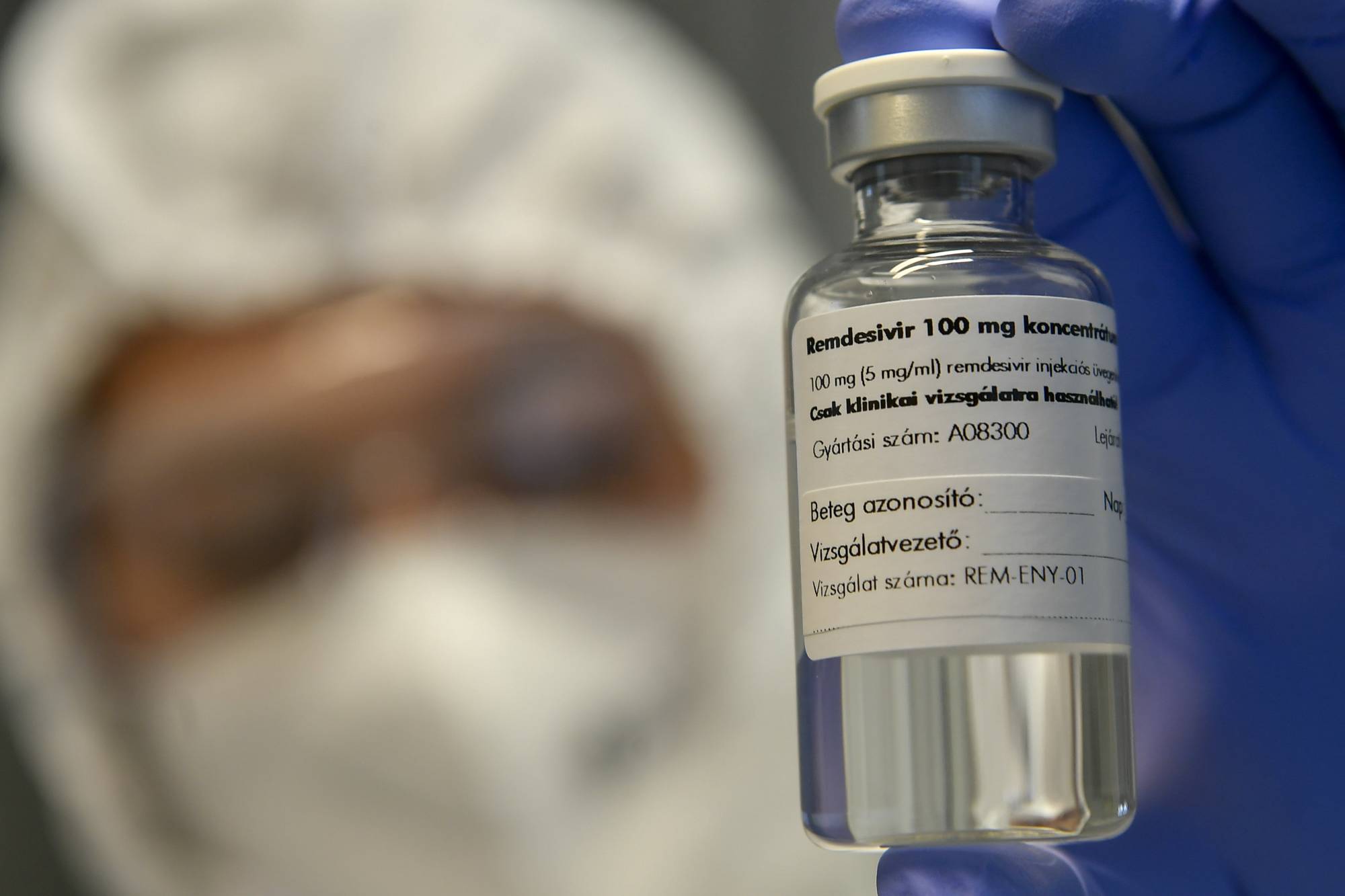
The World Health Organization has recommended against using Gilead Sciences Inc.’s remdesivir to treat hospitalized COVID-19 patients less than a month after U.S. regulators granted the drug a speedy approval.
"There is currently no evidence that it improves survival or the need for ventilation,” a panel of WHO-convened experts developing COVID-19 treatment guidelines said in The BMJ medical journal.
The recommendation is a blow to Gilead’s drug, which was one of the first thought to offer a meaningful benefit in treatment of coronavirus patients after a study showed it reduced their recovery time. The antiviral has been used widely to treat COVID-19 and was among the drugs U.S. President Donald Trump received when he was diagnosed with the disease in early October.

















With your current subscription plan you can comment on stories. However, before writing your first comment, please create a display name in the Profile section of your subscriber account page.