Japan has bolstered its arsenal as it races to catch up to other developed nations and inoculate its citizens against COVID-19 amid a fourth wave of infections.
Two more coronavirus vaccines are set to get the green light from a health ministry panel on Friday as Japan aims to beef up its lagging vaccine rollout.
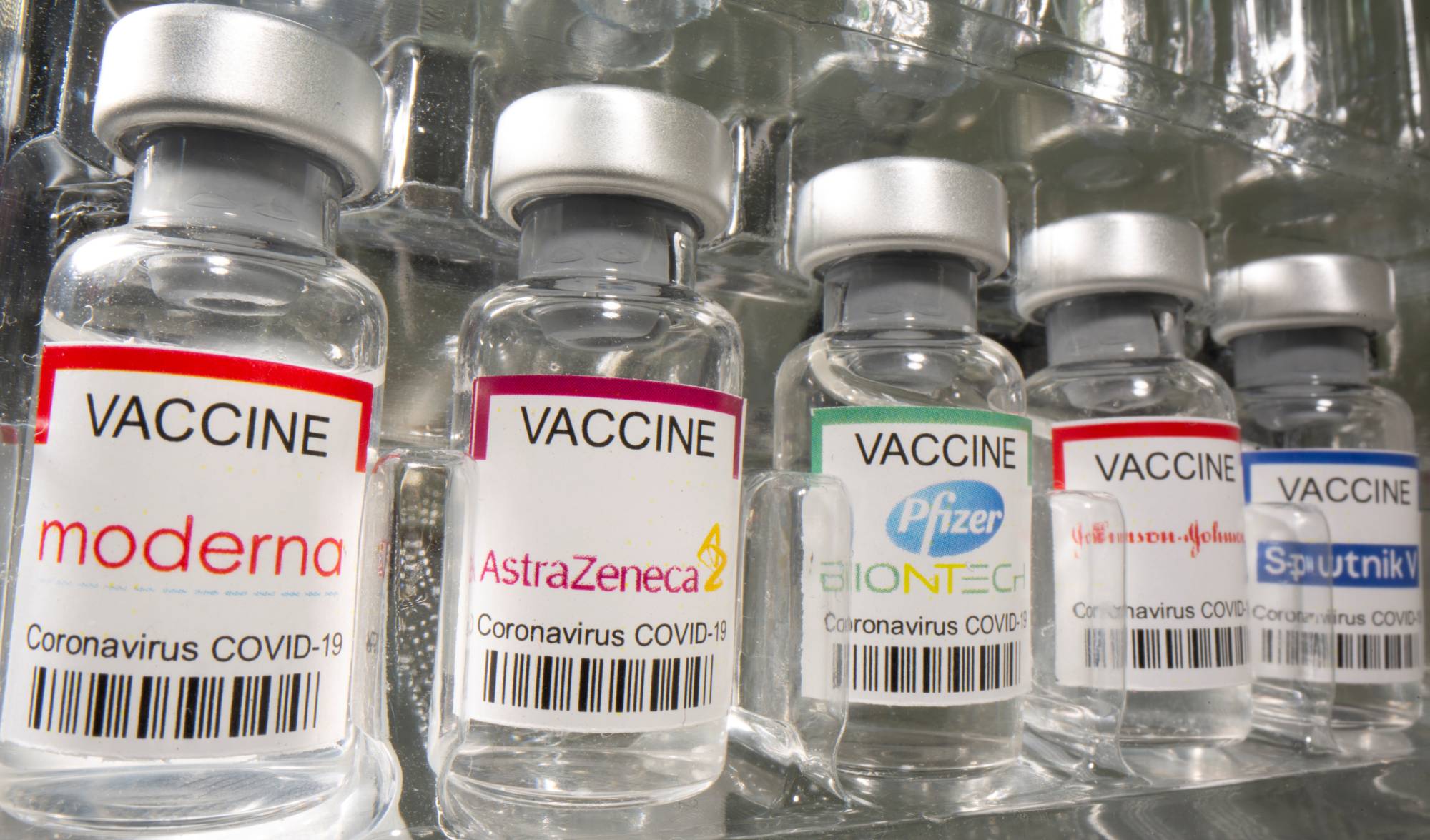
A separate panel of experts on Thursday evaluated the effectiveness and safety of the vaccine developed by AstraZeneca PLC and the University of Oxford and another developed by U.S. drugmaker Moderna Inc. Now that the panel has given its approval, the government will be able to give the shots the OK for emergency use on Friday. Prior to the two new approvals, the vaccine developed by Pfizer Inc. and BioNTech SE is the only jab being administered in the country.


















With your current subscription plan you can comment on stories. However, before writing your first comment, please create a display name in the Profile section of your subscriber account page.