The U.S. Food and Drug Administration is investigating whether the popular heartburn drug Zantac causes carcinogens to form in the bodies of users, in an effort to fully understand the risks posed by the already recalled drug, the agency's spokesman said on Thursday.
The issue of whether ranitidine, commonly known as Zantac, causes levels of the probable carcinogen N-nitrosodimethylamine (NDMA) to rise in users' bodies has been raised previously by Valisure, an online pharmacy that originally flagged the potential contamination of ranitidine to the FDA.
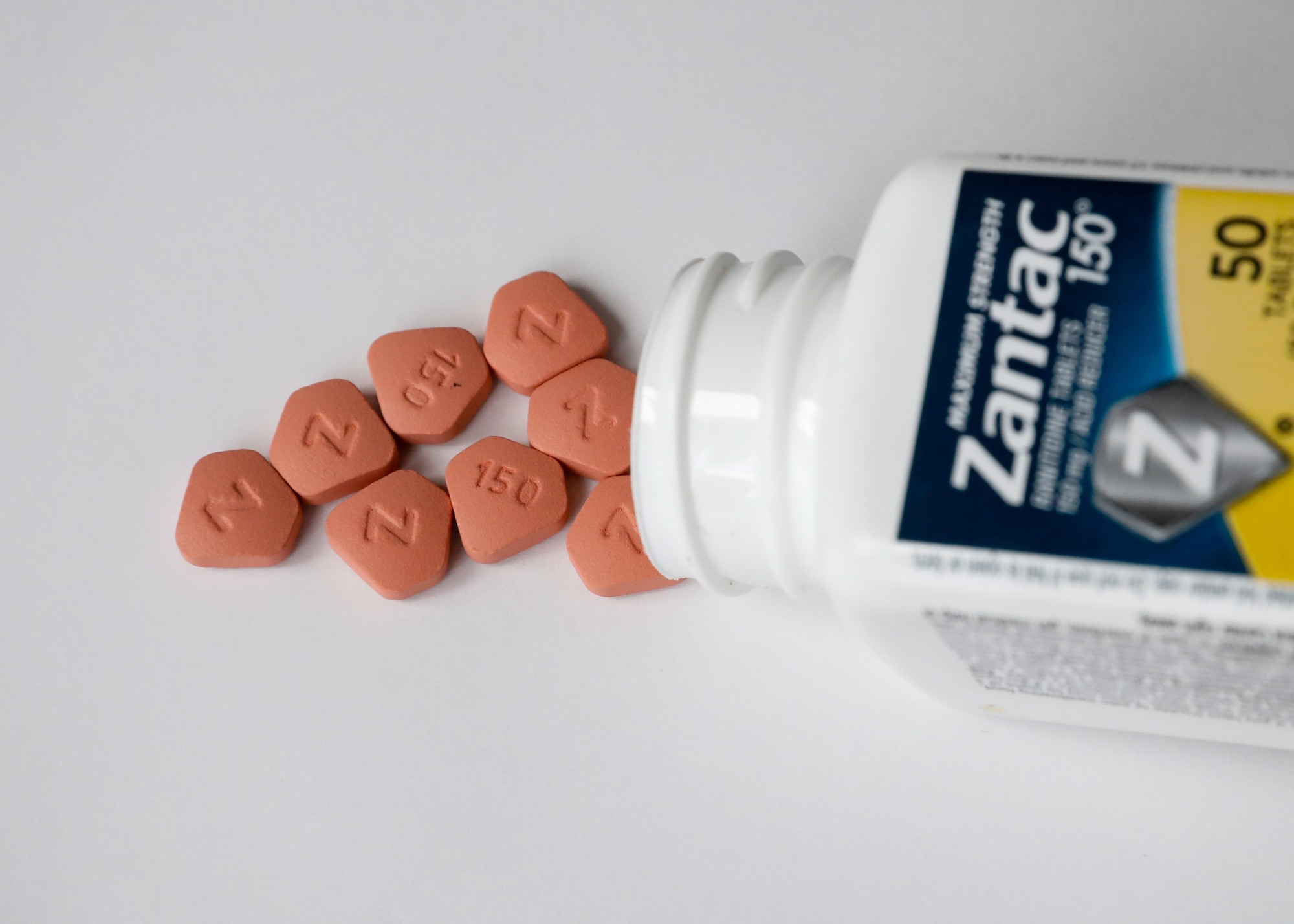
Zantac, sold over-the-counter in the United States by French drugmaker Sanofi SA, and some of its generic versions, have been recalled due to possible NDMA contamination of pills that had not yet been consumed. The FDA said earlier this month it found unacceptable levels of NDMA in drugs containing ranitidine.
But FDA spokesman Jeremy Kahn said the regulator is now "working to understand what happens to NDMA levels in the body, after ranitidine has been exposed to acid in the stomach."
Zantac has been on the market for more than 35 years and was originally sold by Glaxo Holdings Ltd, now a part of GlaxoSmithKline PLC. At one point it was the top-selling drug in the world.
Representatives of GSK and Sanofi were not immediately available for comment.

















With your current subscription plan you can comment on stories. However, before writing your first comment, please create a display name in the Profile section of your subscriber account page.