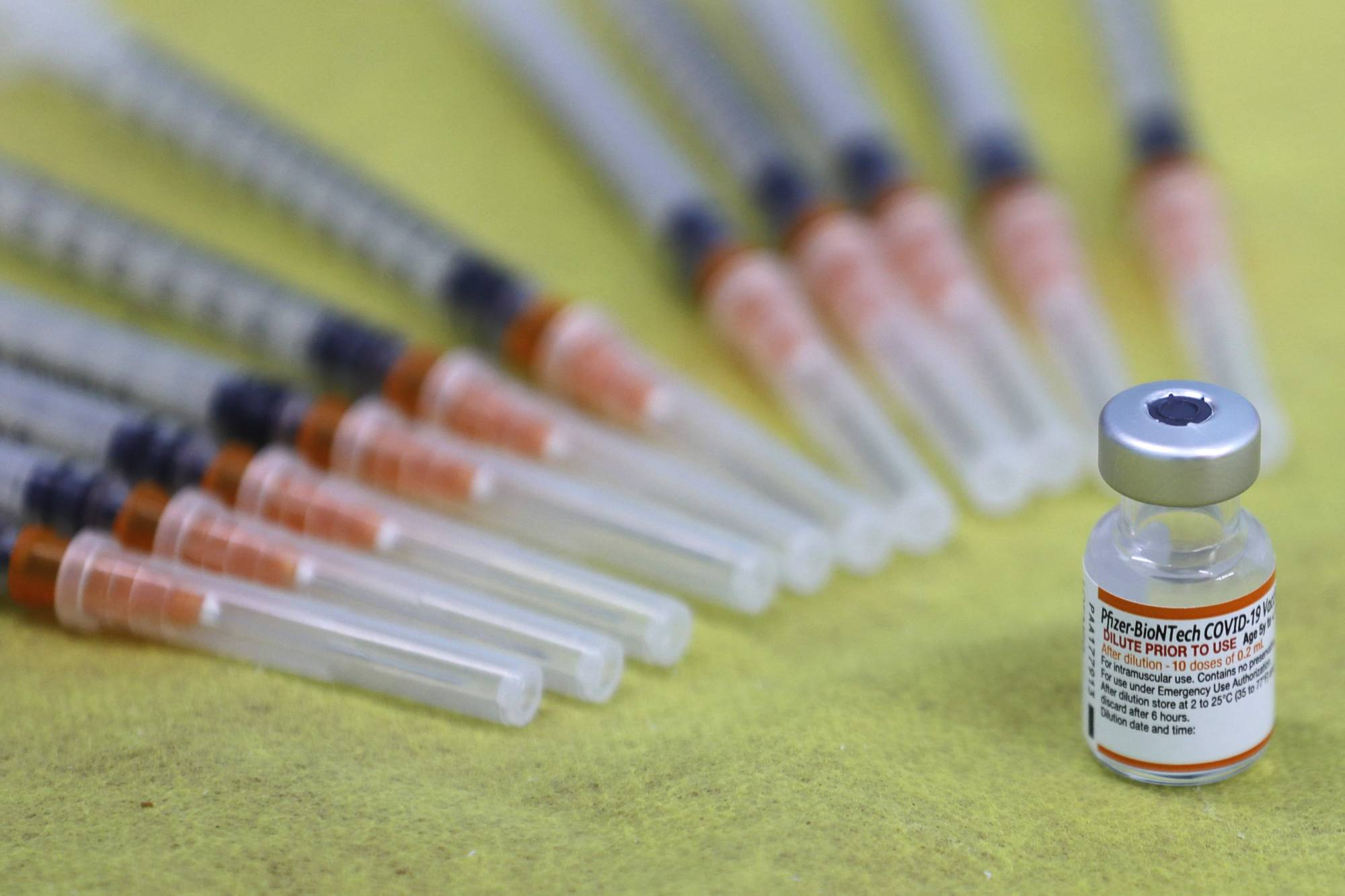
As COVID-19 started spreading in Wuhan early last year, Chinese billionaire Guo Guangchang’s drugmaker appeared to have scored a big win: A partnership with Germany’s BioNTech SE, which went on to produce with Pfizer Inc. one of the world’s most successful vaccines against the coronavirus.
Yet almost a year later, the shot is yet to be approved in mainland China, and in recent weeks Beijing has thrown its heft behind a homegrown mRNA vaccine, allowing China’s Walvax Biotechnology Co. to test its own experimental shot as a booster. The developments are raising new questions about whether the U.S.-German vaccine, licensed for the potentially lucrative Greater China region by Guo’s Shanghai Fosun Pharmaceutical Group Co., will ever be used on the mainland, where President Xi Jinping’s administration has backed a nationalist agenda on all fronts, including in the fight against the virus.
The delayed approval is the latest sign of how vulnerable Chinese tycoons — and their foreign partners — are to Beijing’s political dictats. It also highlights the uncertain outlook for global drugmakers in China, the world’s second biggest pharmaceutical market and a big one for COVID-19 products. More than 1 billion Chinese have been inoculated with the traditional shots made by Sinovac Ltd. and Sinopharm Group even though they have been found to be less effective than mRNA ones in scientific studies.
















With your current subscription plan you can comment on stories. However, before writing your first comment, please create a display name in the Profile section of your subscriber account page.