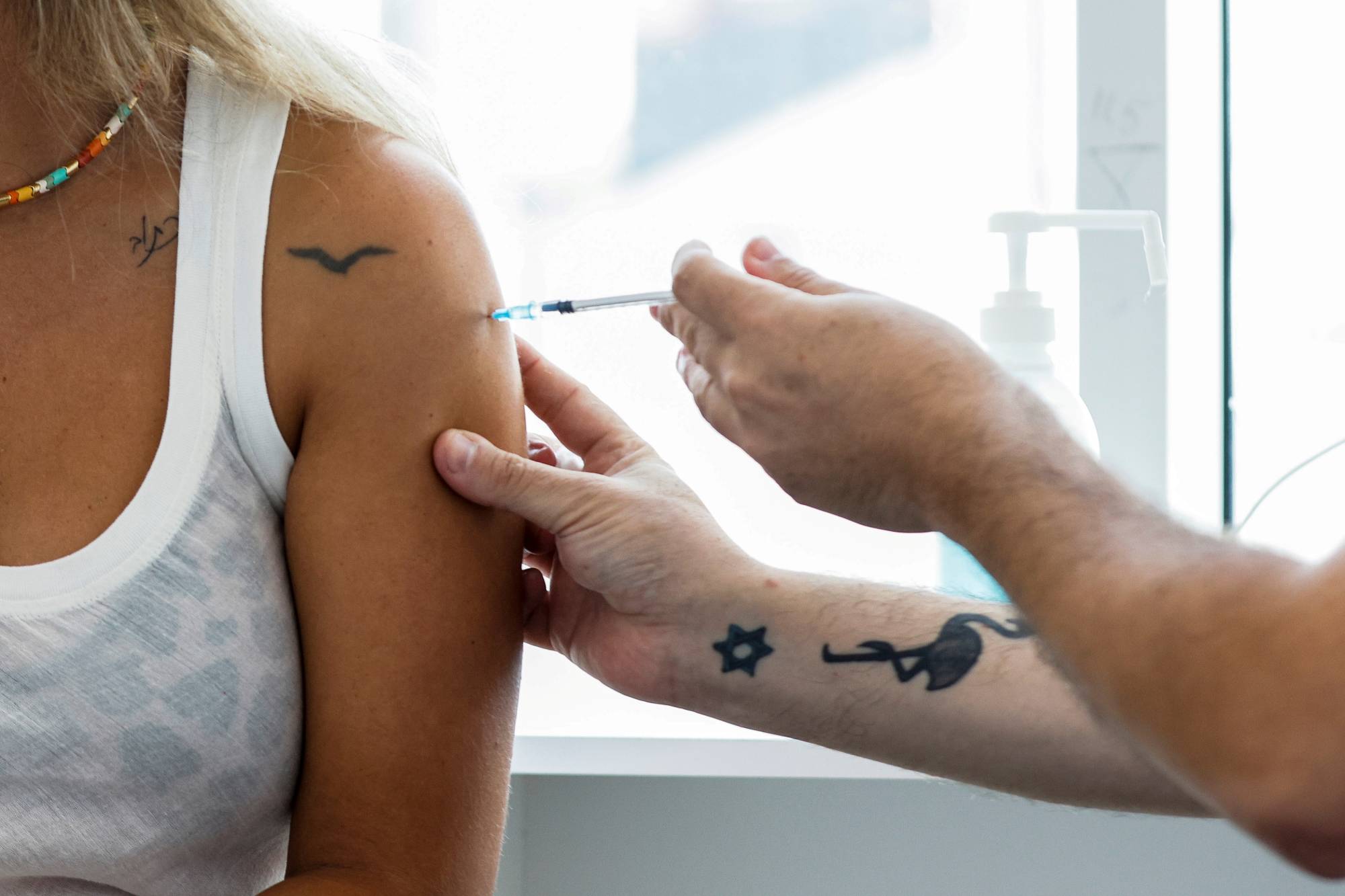
Advisers to the U.S. Food and Drug Administration are expected to discuss two key questions when they meet on Sept. 17 to consider a COVID-19 vaccine booster campaign this fall: Is protection from the initial shots waning, and will boosters help?
The debate will likely be heated following the Joe Biden administration's announcement last month — before the experts could weigh in — that the U.S. plans to start booster doses Sept. 20 if regulators approve them.
The White House move usurped the normal process in which the FDA and the U.S. Centers for Disease Control and Prevention make these sort of science-based decisions, according to interviews with six current and former FDA scientists and CDC advisory panel members.
















With your current subscription plan you can comment on stories. However, before writing your first comment, please create a display name in the Profile section of your subscriber account page.