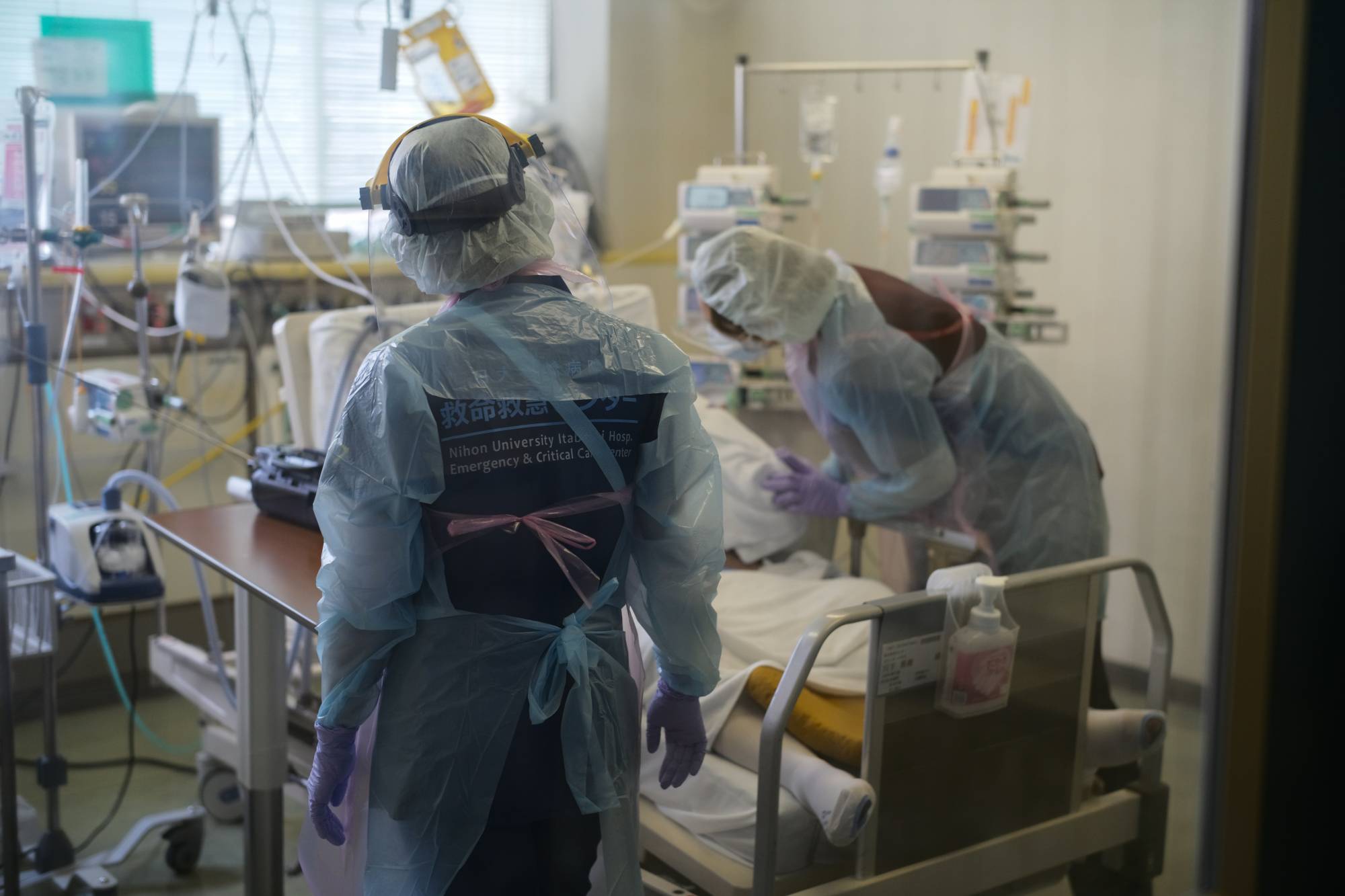
Prime Minister Yoshihide Suga’s administration is planning to use the nation’s first approved drug for mild to moderate COVID-19 cases to treat nonhospitalized patients, in addition to the inpatients currently receiving it.
At the moment, the monoclonal antibody cocktail, which needs to be administered within seven days of the patient developing symptoms, has only been authorized for hospitalized patients.
But with COVID-19 cases rising quickly nationwide and amid increasing calls for its use from medical professions, Suga said last week that the government would work to make what he described as a "revolutionary" drug available to patients quarantined at home.
















With your current subscription plan you can comment on stories. However, before writing your first comment, please create a display name in the Profile section of your subscriber account page.