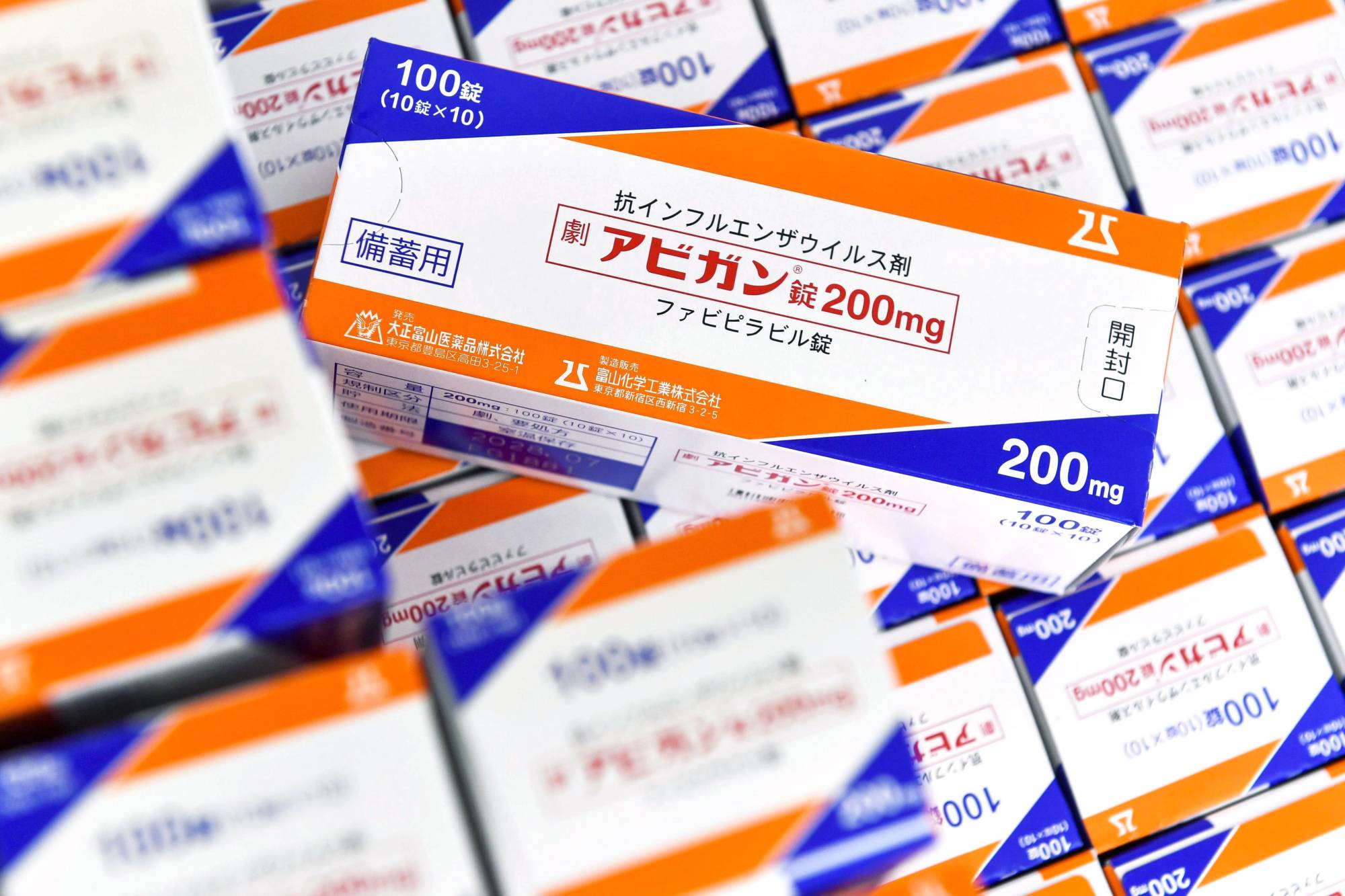
The government is planning to fast-track the approval of anti-viral drug Avigan as a COVID-19 treatment in November even before its developer has filed for a regulatory review, government sources said Saturday.
Under that plan, the review of the influenza drug Avigan as a new coronavirus treatment will be completed in an unprecedentedly short screening period of three weeks once Fujifilm Toyama Chemical Co. submits a new drug application, expected in mid-October, the sources said, raising concerns about whether its safety and efficacy will be thoroughly scrutinized.
Despite some concerns about the drug's side effects, including birth defects, the government has been eager to get the health ministry to approve it as a coronavirus treatment and offer it to other countries.