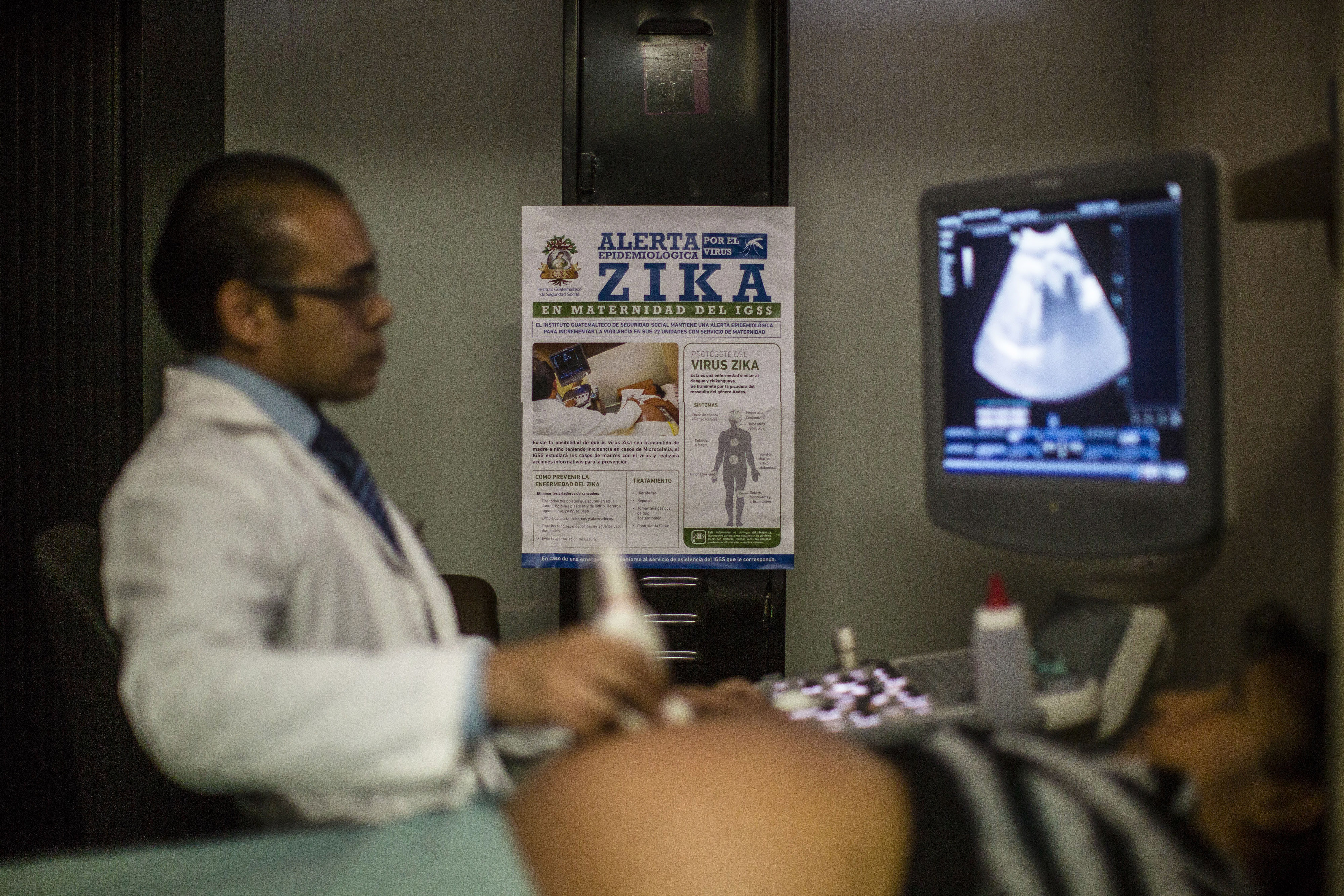
The Zika virus could spread to Africa, Asia and Southern Europe, the World Health Organization said on Tuesday, as major French drugmaker Sanofi SA and others joined the race to create a vaccine.
A day after Geneva-based WHO declared an international public health emergency due to Zika's association with the birth defect microcephaly in Brazil, the United Nations agency said it had launched a global response unit to fight the mosquito-borne virus that is spreading rapidly in Latin America.
Babies born with microcephaly have abnormally small heads and improperly developed brains.