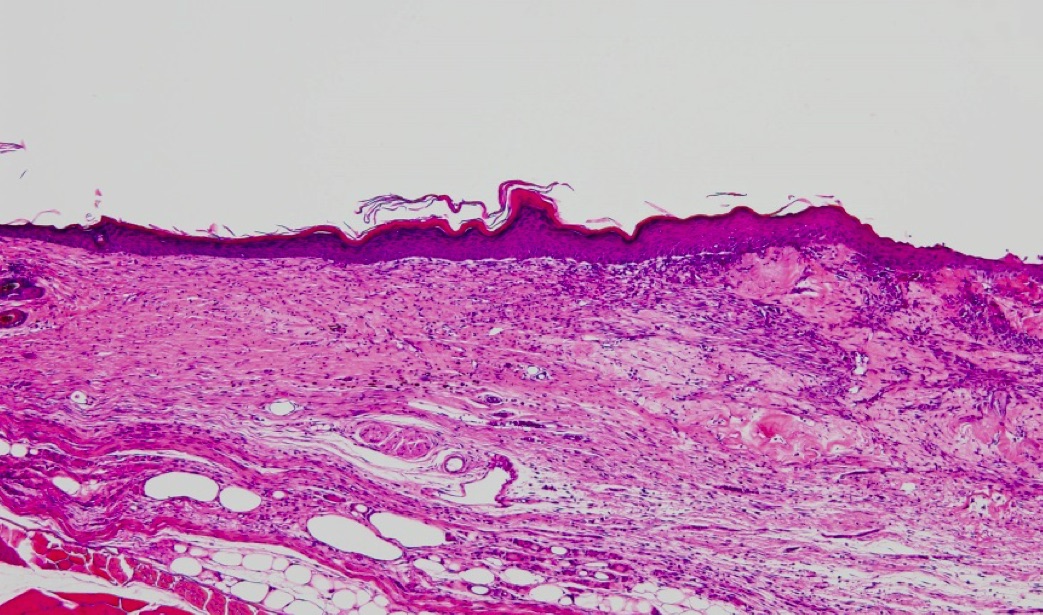
Researchers said Thursday they have developed an easy-to-use artificial skin that acts like a bandage and could be used as a temporary treatment for patients with severe burns until their undamaged skin can be harvested for grafting.
The new technology — which some say could revolutionize the treatment of burns — uses a collagen membrane scaffold to help heal wounds faster, researchers from Saga University and the National Institute of Agrobiological Sciences told The Japan Times. Their findings were published in the June 4 edition of online medical journal Wound Repair and Regeneration.
“In tests conducted on mice we managed to speed up the healing,” said one of the team members who is affiliated with Saga University.